Defining static electricity, how it occurs, and what induces it
It is a cold winter again, and just like any normal winter, you notice some strange things start to happen. One morning, as you pull off your wool sweater, your hair rises and crackles. Not just that, as you walk out of your room, you feel a sharp snap when you reach for the metal doorknob. Careless as you might be, taking it as a trivial matter, your body isn’t joking about it. In that instant, your body’s electrons were having an elegant dance with the atoms of the doorknob, and in a single moment, a cloud of electric charge, a tiny spark like a pinprick of lightning, was discharged between them. This is what we call “static electricity.”
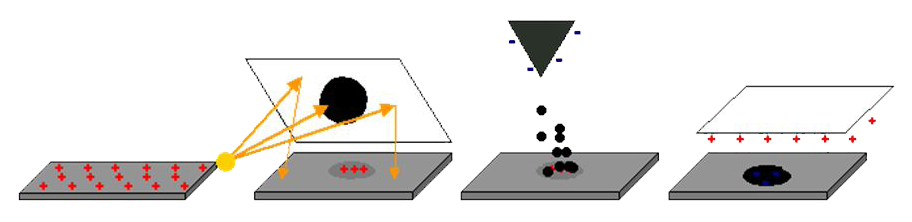
Static electricity, those little shocks and clingy fabrics, may seem magical, but it’s just physics. In simple terms, static electricity is the imbalance of electric charges on surfaces. Normally, atoms have equal amounts of positive (protons) and negative (electrons) charge, which causes most objects to be neutral. Static electricity occurs when this balance is broken, typically through contact or rubbing, which causes electrons to move from one surface to another. In fact, it was the ancient Greeks who first observed and recorded this phenomenon; they noticed that rubbed amber (known as elektron in Greek) attracts straw and other light materials. Seeing this magical effect, electricity was eventually given its name based on that.

The most common way this electron movement happens is through something scientists call the triboelectric effect, though most of us know it simply as rubbing two things together. One classic example is when you rub a balloon against your hair; it’s like you’re playing tug-of-war between the balloon’s atoms and your hair’s atoms, where some atoms are better (stronger) than others. To explain this, scientists even rank materials in a triboelectric series by their tendency to gain or lose electrons, as indicated by the triboelectric series. But the full story is subtle. The balloon’s surface, which has a stronger attraction for electrons than your hair does, pulls electrons away from your hair and onto itself. Your hair, now missing some electrons, becomes positively charged, while the balloon, with its new collection of extra electrons, becomes negatively charged. And after all, what is electricity if not moving electrons? Well, that’s exactly what’s happening here: a small surge of charge we call static electricity.

In fact, recent discoveries have revealed that sometimes, tiny fragments of material transfer between surfaces during contact, not just electrons. Other times, they’re ions, which are atoms that have already gained or lost electrons, that make the journey instead of individual electrons.
The environment plays an important role in this microscopic effect. Humidity acts like a mediator in an argument, allowing charges to dissipate more easily through the moisture in the air. This is why you’re more likely to get shocked in winter when the air is dry and crisp. The water molecules in humid air provide pathways for charges to travel. In dry conditions, these pathways disappear, and charges become trapped on surfaces, building up until they find some path to escape.
Temperature also influences the movement of charges. Heat makes atoms and molecules move more rapidly and randomly. This increased motion can help separate charges or, conversely, help them recombine. Cold conditions tend to preserve static charges longer, which is why that winter sweater seems particularly shocking.
How static electricity affects our daily lives
The effects of static are all around us: the attraction you feel when trying to separate clinging clothes from the dryer, the shock from touching a doorknob, the way a balloon picks up hair after being rubbed, charged dust in a desert storm can spark lightning. These everyday events convey the same physics as a thunderstorm. And this is not an exaggeration. In arid sandstorms, colliding particles build up massive static charges that can eventually discharge as lightning. Even the tiny zap from a doorknob happens when excess charge on your body suddenly finds a path to escape, leaping across the air gap to the metal in a miniature lightning bolt.
Speaking of lightning, these sky-splitting phenomena are just an application of static electricity on a massive scale. Inside traveling storm clouds, ice crystals and water droplets collide and rub against each other, creating charge separation — just like your balloon and hair. However, the difference is that, compared to the few particles involved in balloon-hair rubbing, the rubbing in storm clouds involves trillions upon trillions of particles. The bottom of the cloud typically becomes negatively charged while the top becomes positive, creating a huge electric field. When this field becomes strong enough to overcome the insulating properties of air, electrons leap across the gap, just like in any other case of static discharge, producing the brilliant channels of light we call lightning.
The amount of charge involved in lightning is staggering. A typical lightning bolt carries about 5 billion joules of energy and involves the movement of about 20 coulombs of charge. To put this in perspective with other forms of static electricity, if you could somehow collect all the static charge from every sock coming out of every dryer in a major city for an entire day, you still wouldn’t approach the charge involved in a single lightning strike. Even on a small scale, engineers still exploit static electricity: photocopiers and laser printers literally use static charge to grab ink or toner onto paper in precise patterns. Furthermore, air purification systems use static charges to capture dust and other Volatile Organic Compounds (VOCs), cleaning the air we breathe. Even some manufacturing processes rely on static electricity to position materials precisely in production lines or even to apply coatings evenly.
How Much Blanket Movement to Get Shocked? (Quantitative Analysis)
Imagine that, on another cold winter night, you’re getting ready to sleep. As you pull the blanket over your head, ready to close your eyes and drift off, you suddenly hear that sharp crackle. We’ve already talked about how this crackle, made of just a few volts, isn’t harmful at all. But the idea of getting hurt one day sticks with you, and you ask:
“What if it wasn’t just a few volts? How much rubbing would it actually take for this to stop being a funny little crackle and start becoming something… dangerous?”
The answer to this will definitely be a shocker. (pun intended heh)
We’ll define “dangerous” as a shock of 30,000 volts, which is roughly the limit before air ionizes over a few millimeters, which is enough to cause burns or damage sensitive electronics.
To break down this problem, we can model the human body like a capacitor, which typically has a capacitance of around:
C =100 pF = 100\cdot10^{-12}Taking the voltage as 30,000V, the energy induced in the capacitor (the human body) is given by:
\begin{align*}
E &= \frac{1}{2}CV^2\\
&=\frac{1}{2}\cdot100\cdot10^{-12}
\cdot30,000^2\\ &= 0.045J
\end{align*}
This is 45 millijoules, a shock large enough to cause real pain, trigger reflexes, and in rare cases, damage microelectronics or cause skin burns. The charge needed to give this energy can then be calculated using the capacitance-voltage relation:
Q=C\cdot V=100\times10^{-12}\cdot30,000=3\times10^{-6}That’s 3 microcoulombs of charge needed. Using a piece of information that each rub under a blanket can generate about 1nC of charge, the number of rubs is then easily calculated.
N=\frac{3\times10^{-6}}{1\times10^{-9}}=3000You’d need around 3,000 significant movements (like sliding your foot across the blanket) to build up enough charge for a 30,000-volt shock, assuming dry air and insulating materials.
Realistically, that would mean constant fidgeting for 10–20 minutes under just the right (or wrong) conditions. But for it to actually become dangerous, all that charge would need to be built up and discharged in less than one second. To make that happen, you’d have to be moving at a speed of around 600 m/s, which is supersonic. Fortunately, that’s something we humans simply can’t do.
Conclusion
And yet, even with all the printers, sensors, and electronics that rely on it, static electricity still hides secrets. Scientists have been studying it for decades, and they still don’t fully agree on what exactly is going on when two materials touch and rub together.
Static electricity might just give you a tickly feeling, but it’s actually a very complicated concept that we can’t fully grasp till today. The next time you hear that little snap or feel your clothes clinging, smile at the underlying science, as you are witnessing countless electrons and ions dancing and sticking in great patterns, a scene that reminds us of how physics is so embedded in our natural world.
Acknowledgment. I’d like to thank Hamza El-Rawy, a former Physics Club mentee, for sharing the idea that initiated this research. His inspiration gave me the push I needed to search deeper into the topic, and I’m grateful for that.